各校閲サービスの校閲結果の違いをお感じください
(同一原稿利用)
※以下のバーをクリックしてそれぞれの校閲結果をご覧ください。
原 文
Fingolimod (FTY720) has shown its effectiveness in decreasing relapses among the Caucasian population with MS.
Efficacy and safety outcome of past three years in a 38 year old female patient with relapsing MS treated with FTY720 is reported.
The patient was prescribed FTY720 1.25 mg once-daily for the past three years. Regular MRI and Gd-enhanced MRI examination was done every three months.
For the above described observational period, the patient has been free of Gd-enhanced lesions, indicating favorable result from fingolimod. Adverse events related to FTY720 included liver enzyme levels, and the increased cases of minor palpitations. Although its relationship to the use to FTY720 is yet unknown, the other event reported includes worsening of past symptoms from the past exacerbations, such as persisting numbness and pain in the limbs, headaches, taste disorder.
This case demonstrated the clinical efficacy of fingolimod in reducing relapses in remitting MS patient, consistent with the established effects of fingolimod in Caucasian patients. However, this study indicated the further needs to study the cause and the mechanisms of adverse effects that may not seem directly related to use of FTY720.
カジュアル校正の納品原稿サンプル
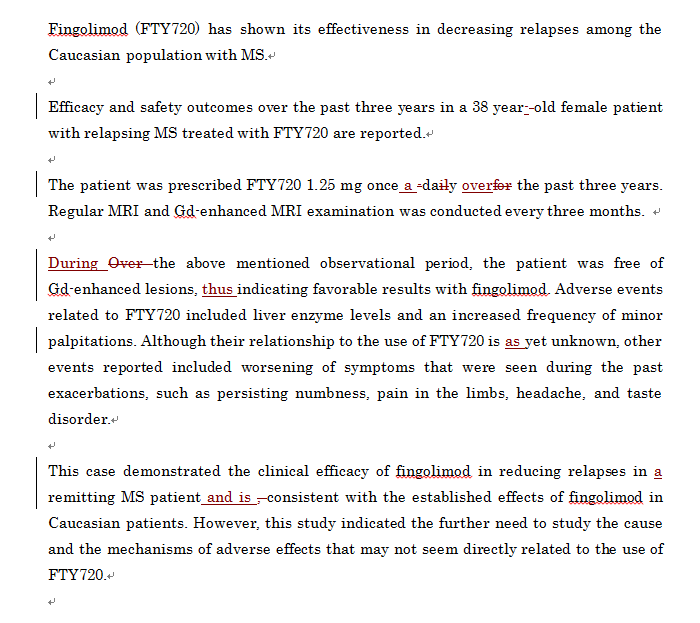
プレミアム校閲の初回校閲納品原稿サンプル
プレミアム校閲では、原文のスタイルを残した上で、文法・言い回し・単語の選択・ロジックなどを査読経験や論文執筆経験豊富な欧米のネイティブ校閲者が校閲します。


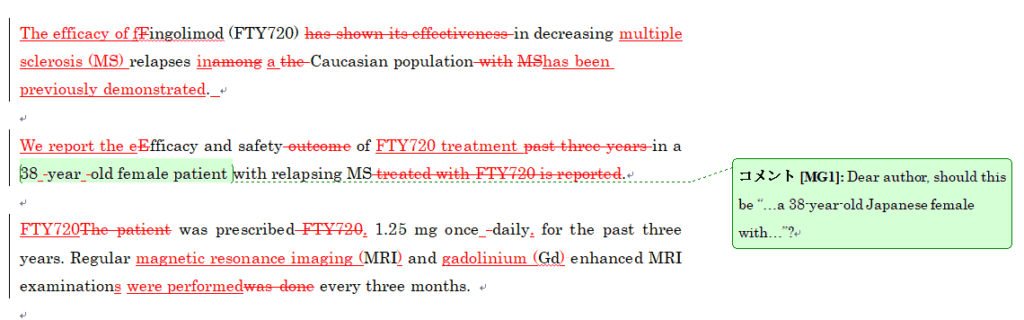
プレミアムEX校閲の初回校閲納品原稿サンプル
プレミアムEX校閲では、プレミアム校閲の校閲に加え、原文のスタイルを残しながらも大幅に書き直す作業を行います。必要に応じて、文の結合や分離等のご提案をさせていただきます。
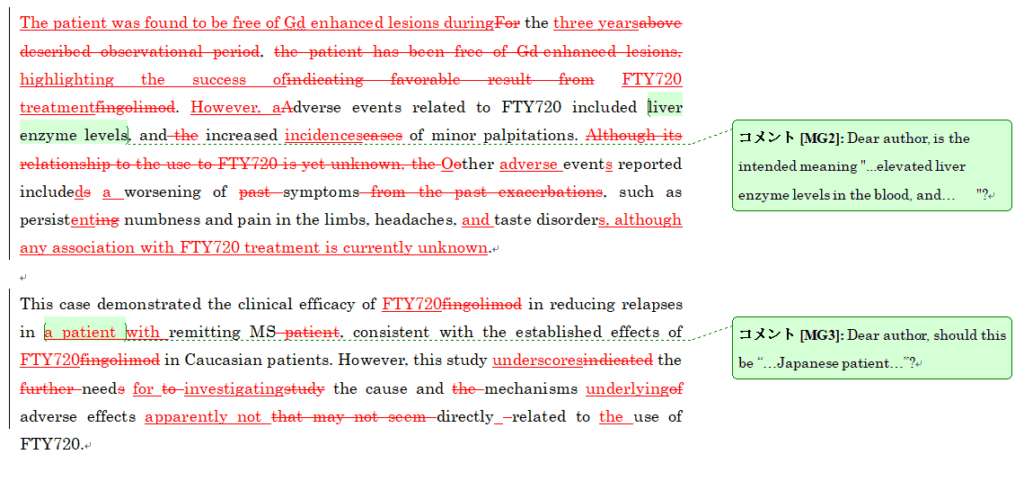
フルチェンジリライトの初回校閲納品原稿サンプル
フルチェンジリライトでは、ネイティブの英語と同等な英文に仕上げるため、文法・語彙選択・論文内容の校閲にとどまらず、原文のスタイルや構成等を根本的に書き換えるリライトを行います。
Purpose: Fingolimod (FTY720; trade name Gilenya, Novartis) is a sphingosine-1-phosphate structural analogue used to prevent autoimmune reactions. It has been shown to decrease relapses among the Caucasian population with multiple sclerosis (MS). The purpose of this study was to determine the efficacy and safety of FTY720, for a prior three year treatment period, in a 38-year-old female patient with relapsing MS.
Methods: The patient was prescribed FTY720 at 1.25 mg/day for three years prior. Routine magnetic resonance imaging (MRI) and gadolinium-enhanced MRIs were conducted on the patient every three months.
Results: For the observational period of three years, the patient was free of lesions, indicating favorable results from fingolimod treatments. Adverse events related to FTY720 included increased liver enzyme levels, and increased episodes of minor palpitations. Additional symptoms which may not be related to FTY720 treatments included worsening of past symptoms, such as persistent numbness and pain in the limbs, headaches, taste disorders.
Conclusions: This case study demonstrated clinical efficacy of fingolimod for reducing relapses in a remitting MS patient. The result was in agreement with the reported effects of fingolimod treatments in Caucasian patients with MS. Further studies should be done to determine the cause and the mechanism of the adverse effects that may or may not be related to the use of FTY720.
※実際の納品原稿は修正履歴及びコメント機能と使用した修正が挿入されておりますが、ここでは原文からの書き換えを分かりやすくするため、修正箇所を表示しておりません。
依頼内容(フルチェンジリライト)
- 専用依頼フォームで以下の項目等を確認いたします。
- スペル:
米国・英国・どちらでも可 - 動詞:
受動態・能動態→ 状況に合わせて選択して欲しい。 - 一人称代名詞:
I・We - 原稿のスタイル変更度合い:
しっかり・適度に - 言い回しまたは単語の変更度合い:しっかり・
適度に - 原稿の流れに対する変更度合い:しっかり・
適度に - 投稿規定と照合:希望する(+20%追加料金発生)

